How To Draw Structure From Nmr Data
How to Draw Structures for NMR Prediction
How to Draw Structures for NMR Prediction
These rules were developed to conform with the algorithmic requirements of ACD prediction software (ACD/CNMR and ACD/HNMR) and IUPAC recommendations and, thus, with the algorithm requirements of ACD/Proper name.
General requirements:
ACD software (ACD/CNMR and ACD/HNMR) predicts the spectrum of the bodily structure you have fatigued. It does not have into business relationship:
Tautomeric forms (even most preferable ones);
Formation of complexes and salts (intramolecular, with the solvent or in case of mixtures).
The 3 dimensional representation of a construction is taken into account simply for predicting 3J constants in HNMR spectra. Information technology is non used when chemic shifts are predicted.
Coordinates (as drawn) are but used to determine the configuration of double bonds too as of isomers of amides, oximes, hydrazones and nitrosamines.
Examples
Double bonds
Avoid defining the configuration of a double bail using stereo bonds.
| Incorrect | Correct |
| | |
Algorithms within the software determine the configuration of a double bond using the angle between the single bond of a substitute and the line going through the double bail. If this angle is not 0o, such a structure is defined as a specific isomer: E or Z:
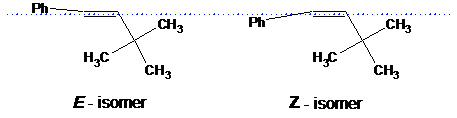
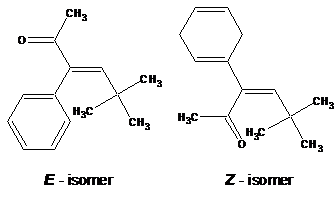
Do not draw ii substituents on one side of a double bond.
The configuration of the double bond in circadian structures should be as shown, with double bonds drawn with Z-configuration (not E-configuration).
| Wrong | Correct |
| | |
Other types of isomerism
Amide isomers should exist drawn with defined substituent positions.
| Wrong | Right |
| (ambiguity is possible) | |
Similar rules should be applied to isomers of oximes, hydrazones and nitrosamines.
Stereo Bonds and Orientation of Substituents
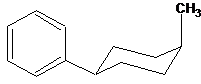
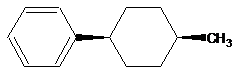
The orientation of substituents should be drawn with the assist of stereo bonds.
| Wrong | Right |
| | |
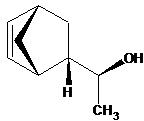
Besides the orientation of substituents, in bicyclic structures it is necessary to show the orientation of a bridge.
Stereo bonds should be designated in such a way that they testify not only the mutual orientation of substituents in a cycle merely likewise the configuration of all chiral carbon centers (and, if possible, of phosphorus, sulfur, etc.).
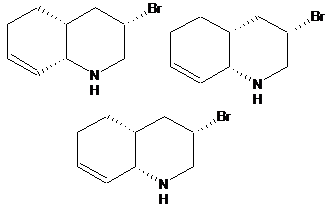
Avoid drawing sequent stereo bonds (�head� to �tail�). In other words, information technology�s preferable to depict two neighboring asymmetric centers so that they are connected with a single bail, but configuration of both of is shown differently.
| Wrong | Right |
| | |
Exercise not use stereo bonds only for the purpose of display.
| Incorrect | Right |
| | |
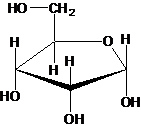
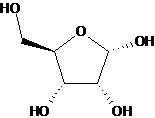
All sugars and their derivatives should be drawn with stereo bonds.
Explicit Hydrogens
For spectral prediction, do not use explicitly fatigued hydrogens, except in some special cases.
Use explicit hydrogens:
To define the configuration of fusion cantlet in the fused cycles:
| Wrong | Right |
| | |
To avoid drawing stereo bonds directly i afterward another:
| Wrong | Right |
| | |
Source: https://www.qreferat.com/referate/chimie/How-to-Draw-Structures-for-NMR928.php
Posted by: turnerfreg1955.blogspot.com








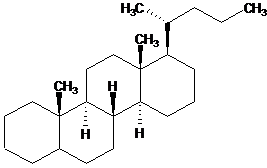
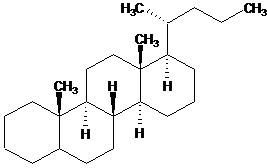

0 Response to "How To Draw Structure From Nmr Data"
Post a Comment